As the safety profile of this new addition to the mCRPC treatment landscape may be unfamiliar to clinicians and patients, we summarize the data from the literature and provide practical guidelines for the management of treatment-emergent adverse events (TEAEs) that may occur during rucaparib treatment.. not be limited to the definition and data, but other factors like study design, explanation in SAP, derivation rules for partial dates should be kept in consideration.. (E9) a treatment-emergent adverse event is defined as an event that emerges during treatment having been absent pre-treatment, or worsens relative to the pre-treatment state..

Summary of treatmentemergent serious adverse events Download Scientific Diagram

Treatmentemergent adverse events Download Scientific Diagram

Treatmentemergent adverse events reported in (a) a phase II trial and... Download Scientific

Treatmentemergent adverse events by severity Download Table

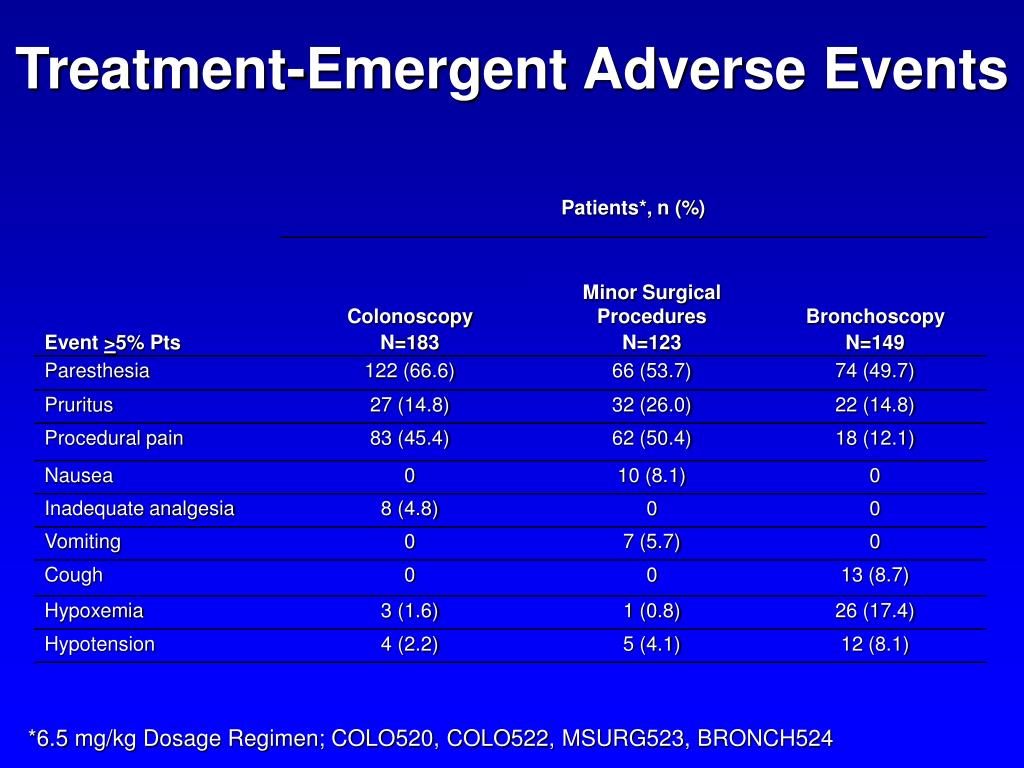
Treatmentemergent Adverse Events Download Table

Study design. TEAE, treatmentemergent adverse event. Download Scientific Diagram

Most Common TreatmentEmergent Adverse Event Occurrences (Q3 in Any... Download Table

Treatmentemergent adverse events (TEAEs; >5 in Download Table
PPT Clinical Safety of Fospropofol Disodium During Diagnostic and Therapeutic Procedures

Summary of TreatmentEmergent Adverse Events Download Table

Adverse Events Report

Treatmentemergent adverse event. Download Scientific Diagram
Adverse effect

Treatmentrelated adverse events occurring in ≥20 patients (N=30) Download Scientific Diagram

Overview of Treatment Emergent Adverse Events (All Randomized Participants) Download Table

PPT Preventing Medical Errors A Team Approach PowerPoint Presentation ID1792249

Treatmentemergent adverse events (TEAEs) and treatment related* TEAEs... Download Scientific

Treatmentemergent adverse events Grade 3 and 4 by SOC and preferred term. Download Scientific

Summary of TreatmentEmergent Adverse Events and Adverse Events of... Download Scientific Diagram

TreatmentEmergent Adverse Event Download Scientific Diagram
Treatment Emergent Adverse Event, TEAE, defines as "an event that emerges during treatment, having been absent pretreatment, or worsens relative to the pretreatment state" according to the E9 guideline. In crossover clinical trials, TEAE can be more complicated due to several factors such as occurrence in washout period, severity change and.. Definitions and Standards for Expedited Reporting). 1.2. Adverse Event (AE) Any untoward medical occurrence in a patient or clinical investigation subject administered a pharmaceutical product and which does not necessarily have a causal relationship with this treatment.